Clinical Evidence
Millions of in vitro diagnostic tests (IVDs) are performed every day to rule out, detect, confirm, and manage diseases and conditions. While much of what is usually seen from IVDs is little more than sample containers, test tubes, transport boxes and perhaps high-end analyzer machinery, it is good to remember that a report from an IVD diagnostic test – be it performed at the bedside, in the hospital lab, or in molecular biology central lab – is just the tip of the iceberg of innovative science, quality control and logistics translated into daily answers.
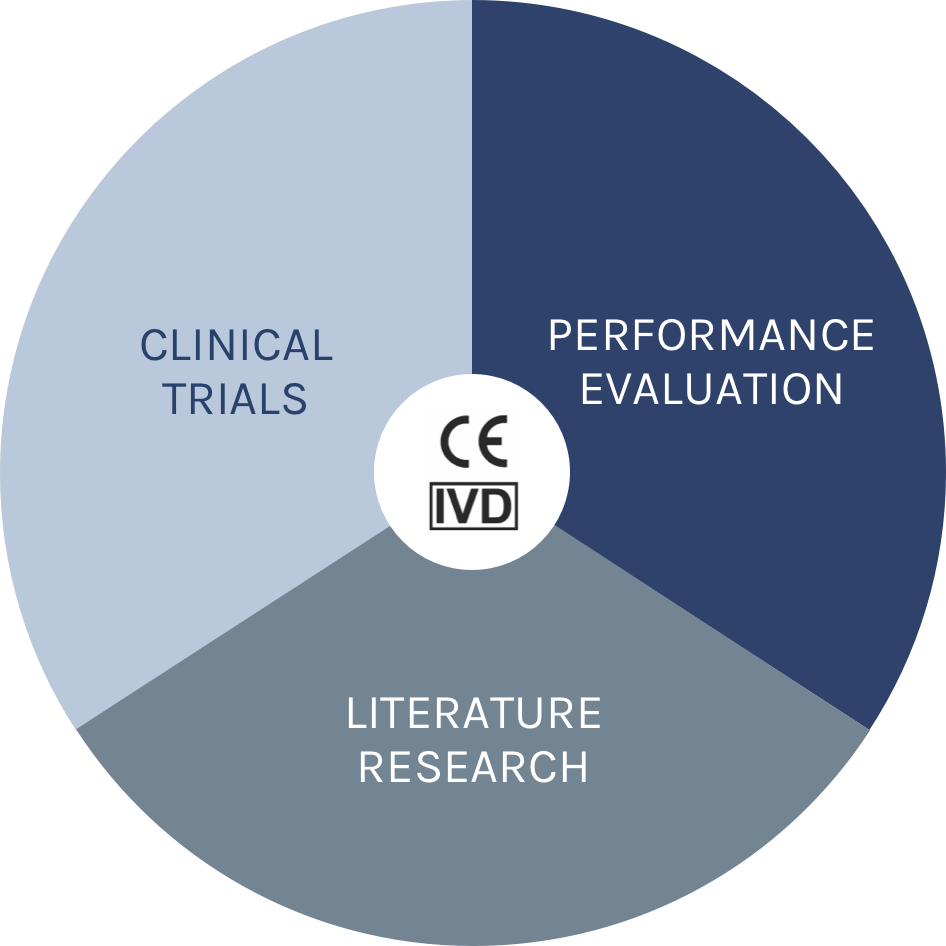
To safely provide these answers, IVDs are heavily regulated by the EU and member states. The CE-mark certification process for every IVD has hence to be conclusive regarding its scientific validity, analytical validity and clinical performance. No IVD test will have a CE-IVD mark unless it meets these demands for clinical evidence.
In other words, clinical evidence is always the sum of clinical trials + literature research + clinical performance = CE-IVD.
Conclusion
Only IVDs which fulfill all of the above requirements may be put on the market and be used by health care providers. Having met these complex regulatory provisions, therefore demonstrating sufficient clinical evidence, IndiTreat® is now being translated into clinical use across Europe. The ever-increasing pool of real-world data regarding 3D microtumor-based testing with IndiTreat® will support the clinicians’ decision making process for therapies in mCRC patients with severe medical needs.